In 1859, Edwin Drake drilled sixty-nine and a half feet into the earth and struck black gold - thus beginning the modern American oil industry. In the 160 years since, such surface oil has been drilled and drained away, forcing the petroleum industry deeper and deeper into subsurface reservoirs than Drake could have ever imagined. Postdoctoral Fellow, Rencheng Dong, at the Oden Institute is helping them get there.
Rencheng Dong is a recent graduate from the Center for Subsurface Modeling, receiving his PhD in August 2021. He remains at the Oden Institute as a postdoctoral fellow in the Center.
His PhD research was picked up and featured in a recent article by SIAM News.
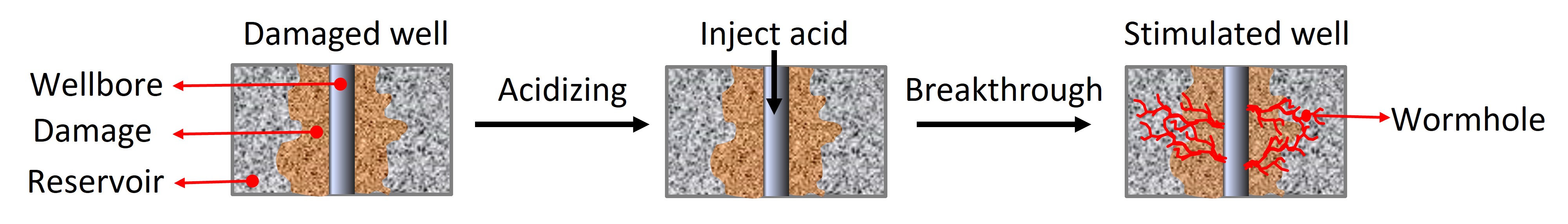
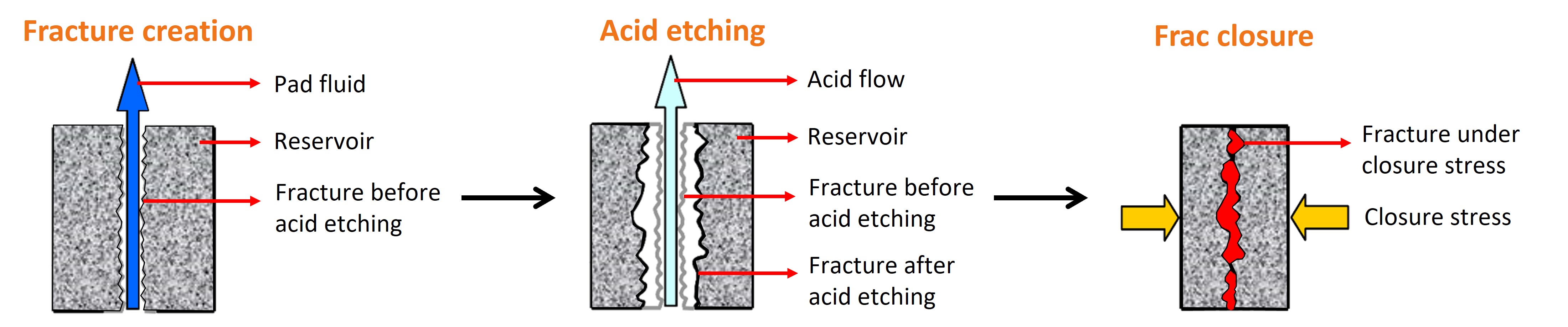
“There are thousands of feet of rock above oil,” said Rencheng. “The more permeable rock is, the better oil flows through it. Initially, the permeability of subsurface rock is very low, but by injecting some acid into the rock we can make its pores bigger, increasing permeability. This is called matrix acidizing.”