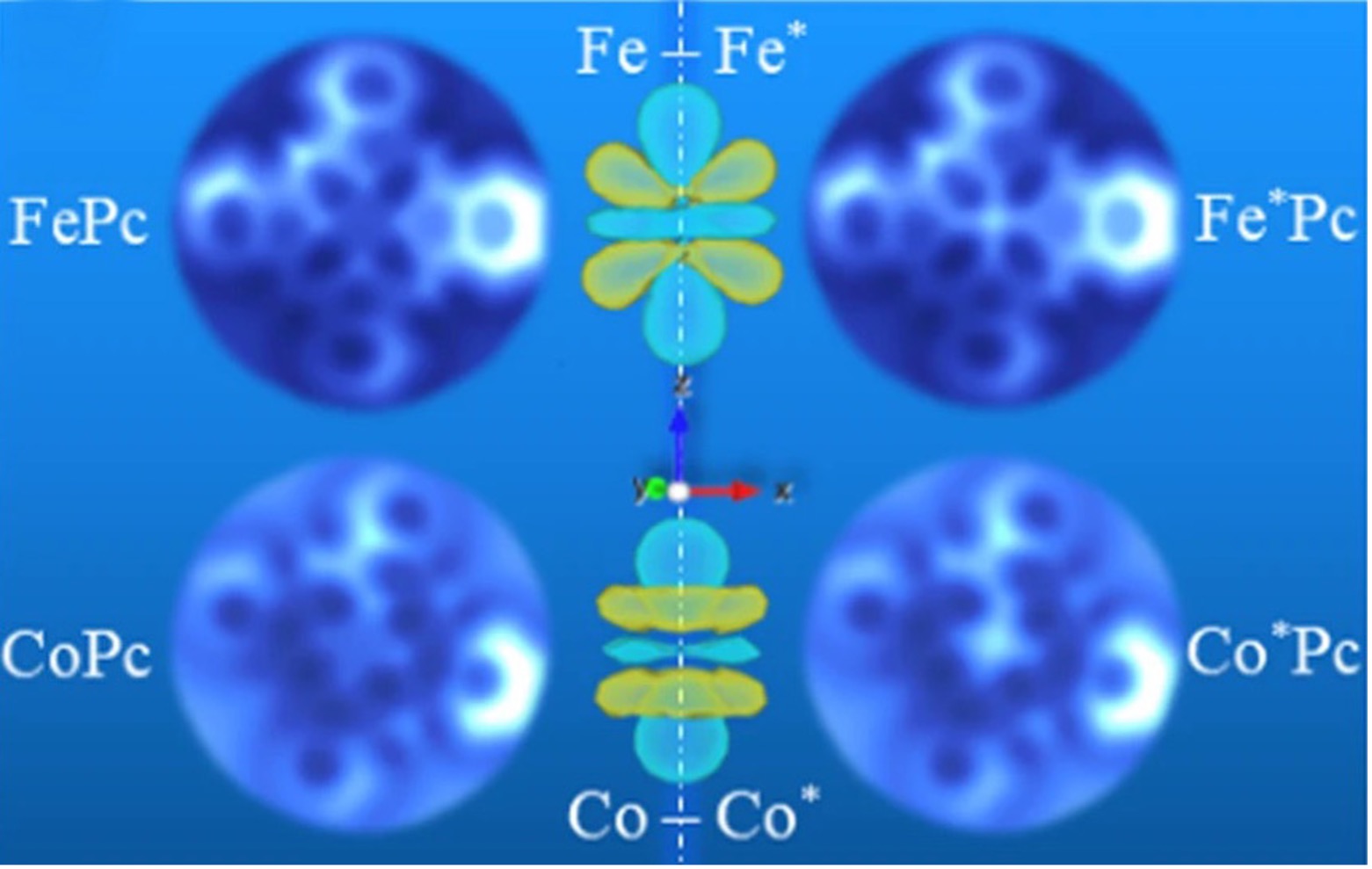
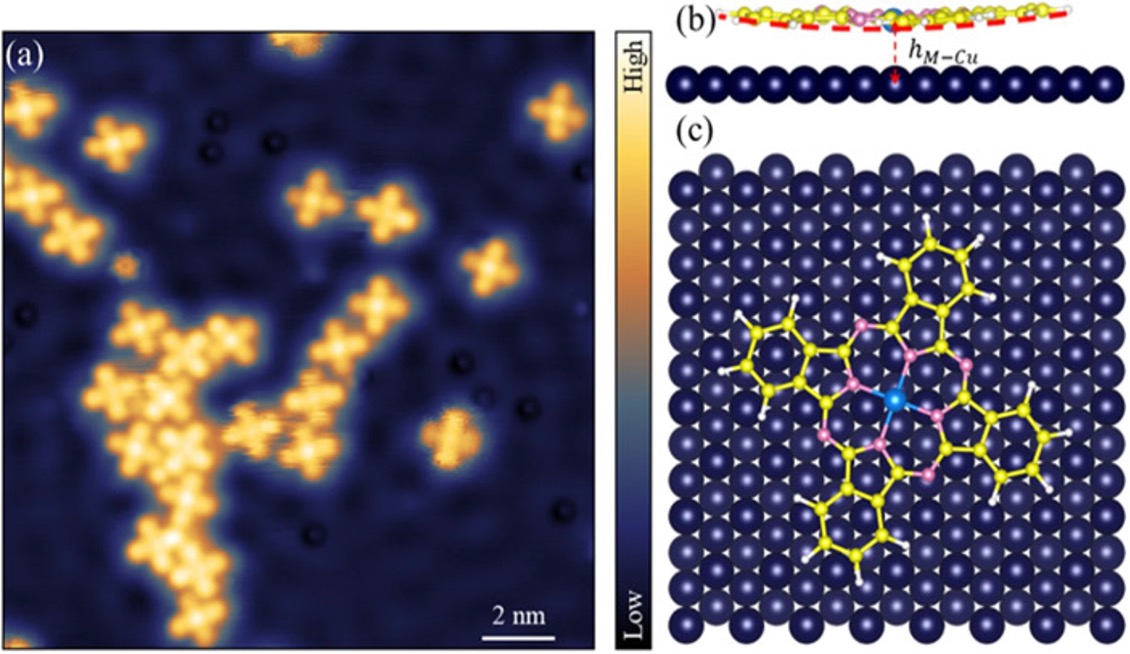
No one will ever be able to see a purely mathematical construct such as a perfect sphere. But now, scientists using supercomputer simulations and atomic resolution microscopes have imaged the signatures of electron orbitals, which are defined by mathematical equations of quantum mechanics and predict where an atom’s electron is most likely to be.
Scientists at UT Austin, Princeton University, and ExxonMobil have directly observed the signatures of electron orbitals in two different transition-metal atoms, iron (Fe) and cobalt (Co) present in metal-phthalocyanines. Those signatures are apparent in the forces measured by atomic force microscopes, which often reflect the underlying orbitals and can be so interpreted.
Their study was published in March 2023 as an Editors’ Highlight in the journal Nature Communications.
“Our collaborators at Princeton University found that despite Fe and Co being adjacent atoms on the periodic table, which implies similarity, the corresponding force spectra and their measured images show reproducible experimental differences,” said study co-author James R. Chelikowsky, the W.A. "Tex" Moncrief, Jr. Chair of Computational Materials and professor in the Departments of Physics, Chemical Engineering, and Chemistry in the College of Natural Sciences at UT Austin. Chelikowsky also serves as the director of the Center for Computational Materials at the Oden Institute for Computational Engineering and Sciences.