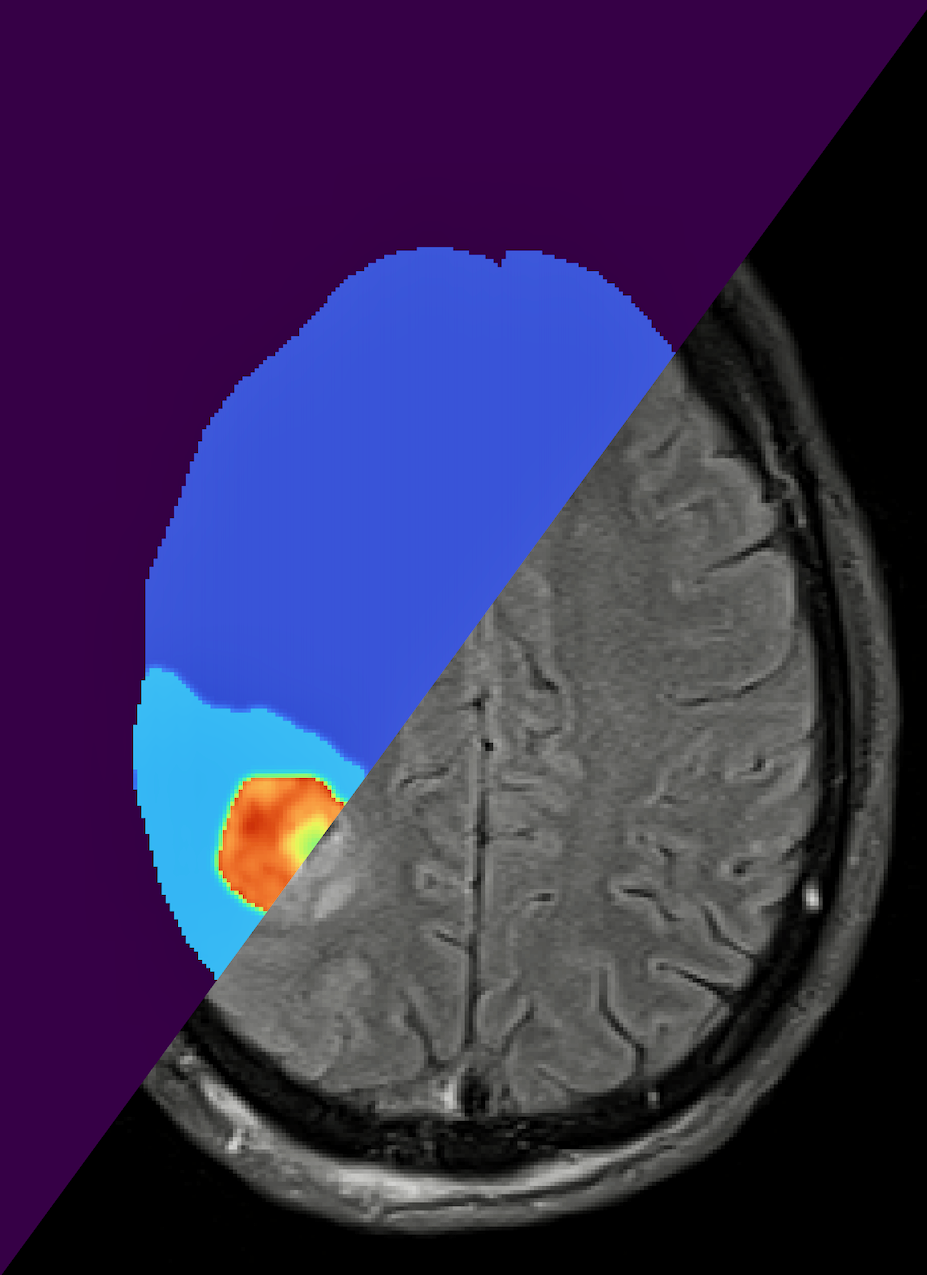
Imagine a world where cancer treatment is not a one-size-fits-all approach. Enter the digital twin, a mathematical model that virtually represents a physical object and the way that object changes — in this case, a brain tumor. Because the digital twin is personalized to the individual patient, it can predict response to treatment and provide doctors with targeted advice.
This isn't science fiction. It's the result of meticulous work by a collaborative team of researchers from the Oden Institute for Computational Engineering and Sciences at The University of Texas at Austin. This collaboration is unusual, bringing together two disparate fields — aerospace engineers from the Willcox Research Group, led by aerospace engineering professor Karen Willcox, with oncology experts from the Center for Computational Oncology, led by biomedical engineering professor Thomas Yankeelov. Published in the journal Frontiers in Artificial Intelligence, their recent paper shows how methods developed for digital twins in aerospace engineering can be used for high-grade glioma patients undergoing radiotherapy.
“This collaboration and this paper highlight one of the very special things about the Oden Institute,” said Professor Karen Willcox. “The Oden Institute is one of the few places in the world where you would find aerospace engineers colliding with biomedical engineers, all bound by the shared connection of using computational science to advance their fields.”
The digital twin is initialized with population-level clinical data and then tailored to an individual patient through Bayesian model calibration, continually assimilating patient-specific magnetic resonance imaging (MRI) data as it is logged in the clinic. Using this data, the digital twin predicts how the patient would respond to different dosages and lengths of radiotherapy. It is the Bayesian framework that sets this research apart. By employing a Bayesian framework, this digital twin accounts for the uncertainty in a patient's data and in their predicted response to treatment. This is essential for making the high-stakes decisions that come with treating cancer.
"There's been a history of building these mathematical models, but it hasn't been until very recently where we could actually personalize these models to individual patients," said David Hormuth, a research scientist at the Center for Computational Oncology.
Hormuth envisions a shift from the current one-size-fits-all radiotherapy treatment plan to a more personalized approach. "Right now, almost every patient that comes in gets the exact same radiotherapy treatment plan. It's just tailored based on the shape and location of the tumor. It doesn't consider how that tumor is actually going to respond to radiation therapy. So, this would provide the clinicians a tool to see, 'Okay, this is our anticipated response to radiation therapy.'"
The researchers tested the methodology by simulating 100 virtual high-grade glioma patients with tumor growth and responses to treatment typically observed in real life patients. Their results show a significant improvement over traditional radiotherapy, delaying median tumor progression by approximately six days. While these are early results with simplified models, they highlight the potential for enhanced patient outcomes.
The digital twin allows for tumor control that is equivalent to the current standard of care but with the use of lower total radiation doses. This works because the digital twin fine-tunes treatment schedules based on the individual patient, so that lower doses can be more effective.
Lower radiation doses mean a better experience for patients fighting cancer. It's getting the right medicine at just the right amount—less side effects, more comfort, and an overall improved journey toward recovery. Moreover, the range of optimal solutions includes alternatives with elevated doses, particularly beneficial for patients with aggressive cancer, as the standard-of-care fails to ensure adequate tumor control in such cases.
"This particular work is aimed towards building trust in a personalized medicine setup through patient-specific digital twins, which is based on predictive modeling rather than just reacting to what you can see," explained Anirban Chaudhuri who is a research associate with the Willcox Research Group and lead author on the paper. "This is our first step towards patient-specific digital twins that support clinicians in their decision making. This is not yet in clinics, but we have plans for future collaborations to get this into the hands of clinicians."
As the researchers work to take this methodology into clinical settings, the impact on patient care could be revolutionary. Advancing digital twins isn't just about better algorithms and medical imaging — it's about providing hope. For cancer patients, it could mean a future where treatment is precisely tailored, where guesswork is minimized, and where the best possible outcome becomes reality.