In the 1950s, the U.S. Air Force tried to design a cockpit to fit the average pilot's body. After measuring 4000 servicemen, they discovered that none of them came close to having the “average” body and any cockpit built for the average pilot would fit no pilot. This led to the rise of adjustable seats.
Researchers at The University of Texas at Austin are tackling the same problem in cancer care. Their recent publication in iScience, titled “Designing clinical trials for patients who are not average,” presents a computational framework tailoring clinical trials to the individual patient.
The Problem with Traditional Clinical Trials
Traditional clinical trials follow a standardized process, testing new interventions on groups of patients to determine safety and efficacy. However, this approach has limitations. It often takes years and significant resources to complete a trial, with only a fraction of drugs gaining approval. Moreover, the one-size-fits-all nature of these trials overlooks many of the differences between individual patients.
Dr. Tom Yankeelov, Director of the Center for Computational Oncology at UT’s Oden Institute for Computational Engineering and Sciences, emphasized that this limitation is particularly pronounced when treating cancer, which is characterized by its heterogeneity. He explained, "Everybody really is different and unique. And so there's this notion that if you design a clinical intervention that works best for the average of the population, then almost by definition, you're designing it to work for nobody, because there is no average."
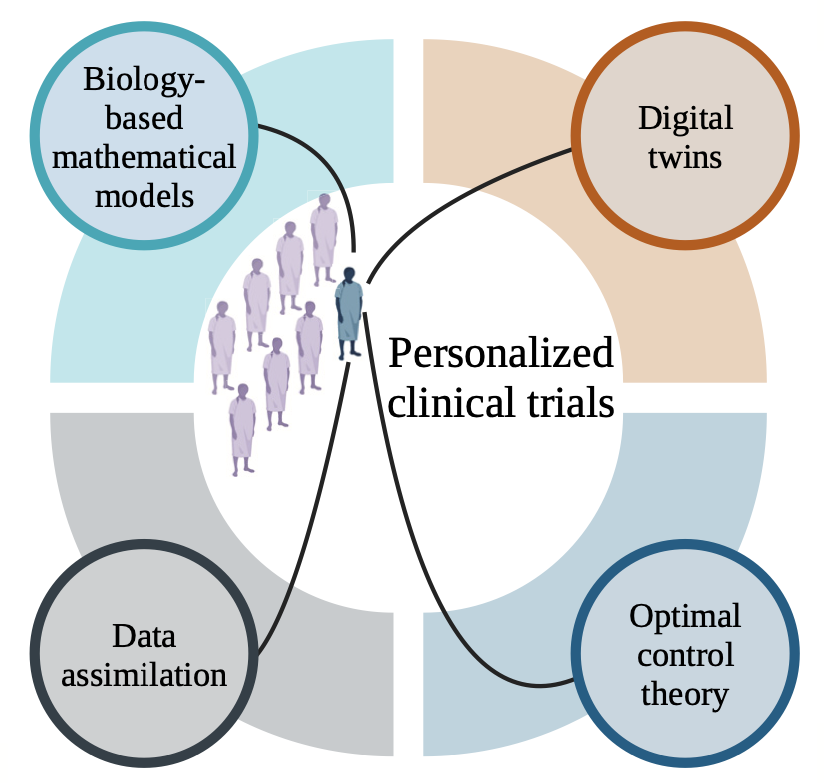
Oden Institute researchers, in collaboration with the University of Texas M.D. Anderson Cancer Center, are advocating for a shift from traditional population-based clinical trials to a more personalized approach - with the help of computational science.
Personalizing The Clinical Trial
Here’s how it works:
Individual patients' tumors would be characterized using imaging techniques and molecular analysis to gather patient-specific data. This data would then be fed into a kind of mechanism-based mathematical model built on the underlying biology and physics of cancer, creating a patient-specific “digital twin.”
This digital twin is a mathematical model that virtually represents a physical object and the way that object changes — in this case, a tumor. Because the digital twin is personalized to the individual patient, it can predict response to treatment and provide doctors with targeted advice.
With the help of this tool, clinicians could simulate different treatment scenarios (chemotherapy vs. radiotherapy, or a smaller dose vs. a larger dose) to optimize their treatment plans for individual patients based on their digital twin’s predicted outcomes. As a patient undergoes the journey of treatment, her model would be updated through data assimilation, ensuring that the treatment plan remains responsive to her evolving condition.
Challenges and Opportunities
While this framework shows promise, challenges remain. Digital twins, while powerful, are still in the early stages of development for cancer care. Similarly, optimal control theory — the mathematical approach behind optimizing factors like dosage or timing to try to help clinicians maximize their effectiveness for each patient — requires further validation in clinical settings.
Dr. David Hormuth, a Research Scientist at the Center for Computational Oncology, underscored the motivation to make these computational models work well for people suffering from cancer, stating, "At the end of the day, you want to balance treatment efficacy with the control of the disease and the reduction of side effects. That may affect the quality of life of the patient."
Currently, clinicians have to estimate the dosage or treatment schedule for a patient based on their professional experience and the results of mass clinical trials and then adjust it after seeing how the patient responds. Too high a dose can mean painful and unnecessary side effects. Too low a dose can allow the cancer to spread. The success of this new computational framework may spare future patients the ordeal of trial and error.
The Future of Clinical Trials and Cancer Care
The rise of the single-subject clinical trial heralds an evolution in medicine. Just as the U.S. Air Force discovered the fallacy of designing cockpits for the "average" pilot, the field of oncology is recognizing the limitations of standardized treatment approaches. Time will tell how the shift from population-based to patient-based may impact the lives of those living with cancer.