On a macroscopic level, exemplified by the sight of an ice cube melting in the heat of a warm Texas summer, physical processes tend to follow the second law of thermodynamics - the idea that the entropy (randomness) of an isolated system always increases.
Computing the entropy production of microscopic molecular systems has become a leading question in the field of physical chemistry. However, in order to do this accurately, researchers must account for the randomness and thermal fluctuation that contribute to microscopic motion, which may allow for certain steps or events that transiently ‘violate’ the second law of thermodynamics, making the directionality of these processes difficult to discern.
New research led by Dmitrii Makarov at the Oden Institute of Computational Sciences and Engineering, in collaboration with researchers from The University of Texas at Austin and the Max Planck Institute for Multidisciplinary Sciences, highlights a new methodology to detect and quantify the entropy production and irreversibility of single-molecule experiments. Published in the Proceedings of the National Academy of Sciences, their paper outlines an innovative approach to quantify irreversibility using available experimental measurements - while other quantification methods typically require information that is not immediately available and more difficult to access.
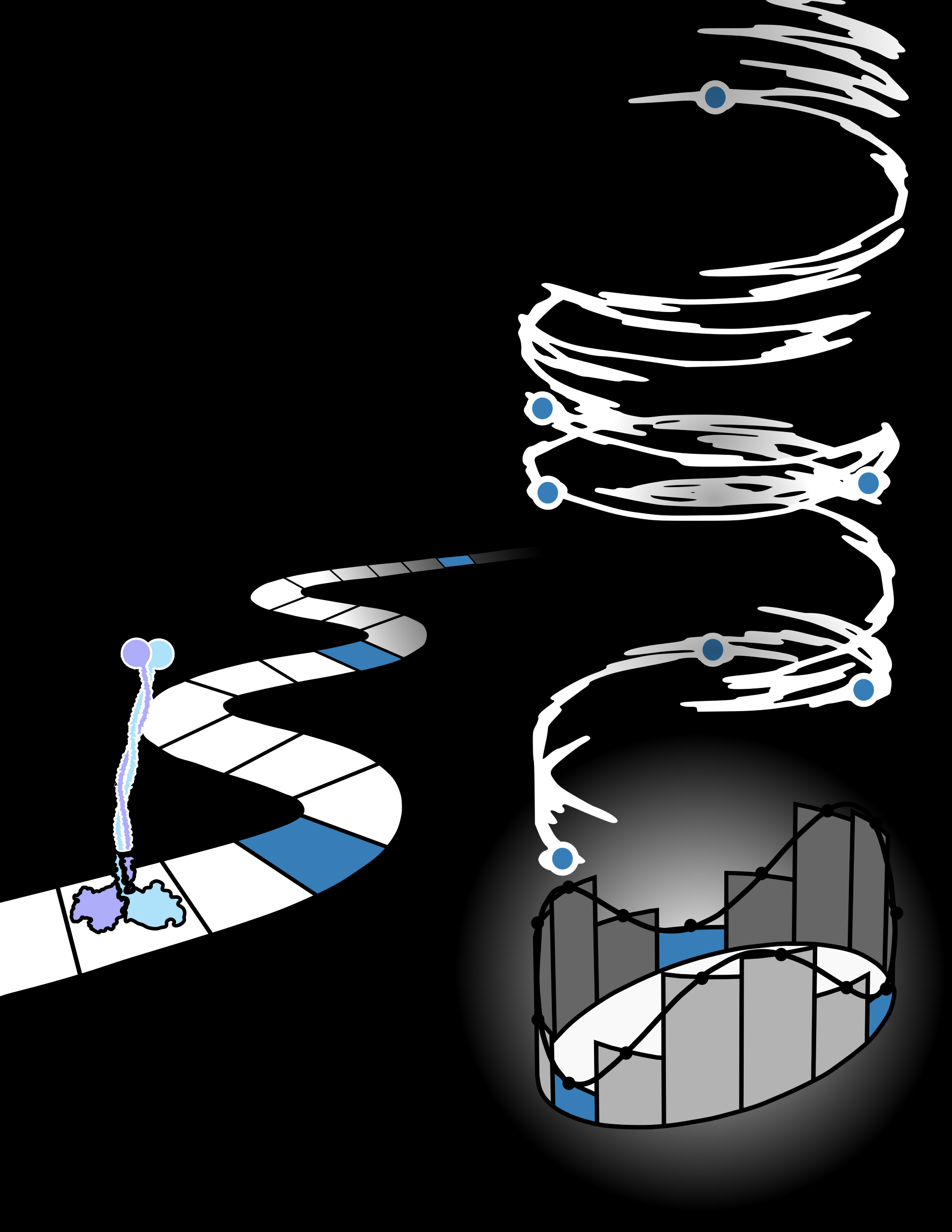
Makarov’s team began exploring new avenues of analyzing single-molecule systems through the lens of information theory, which deals with discrete values that describe the storage and transmission of information. However, when applied to an innately continuous process, such as the diffusion of a gas, it can become difficult to determine where to draw the line in terms of dividing continuous motion into discrete sections.
“This is something that chemists often face when they try to map a continuous molecular configuration onto discrete chemical species,” Makarov explained, describing this discretization in the context of determining “how far apart two hydrogen atoms need to be before they are no longer called a molecule.” This mathematical subjectivity can lead to inaccuracy and increased error in thermodynamic inferences based on experimental data.
Milestoning, invented by Ron Elber, director of the Center for Computational Life Sciences and Biology, circumvents this problem by zooming out of a highly discrete perspective, and outlines a computational framework for interfacing with continuous processes. Based on this collaboration, Makarov’s team applied milestoning in the context of non-equilibrium processes, and found that thermodynamic inferences can be made more accurately if some elements of microscopic behaviour are ignored. Eloquently described by the title of their paper, they have shown that “ignorance is bliss” when making thermodynamic estimates - based on their work, it’s clear that blurring and zooming out leads to increased accuracy.