Michael Sacks, Director of the Willerson Center for Cardiovascular Modeling and Simulation at the Oden Institute and his team tackled this puzzle by creating a detailed computer model of the heart.
“This is a result of about thirty years worth of work by myself and my collaborators on trying to understand how myocardial infarction and mitral valve regurgitation happen together,” said Sacks, who is also a biomedical engineering professor at the UT Cockrell School of Engineering.
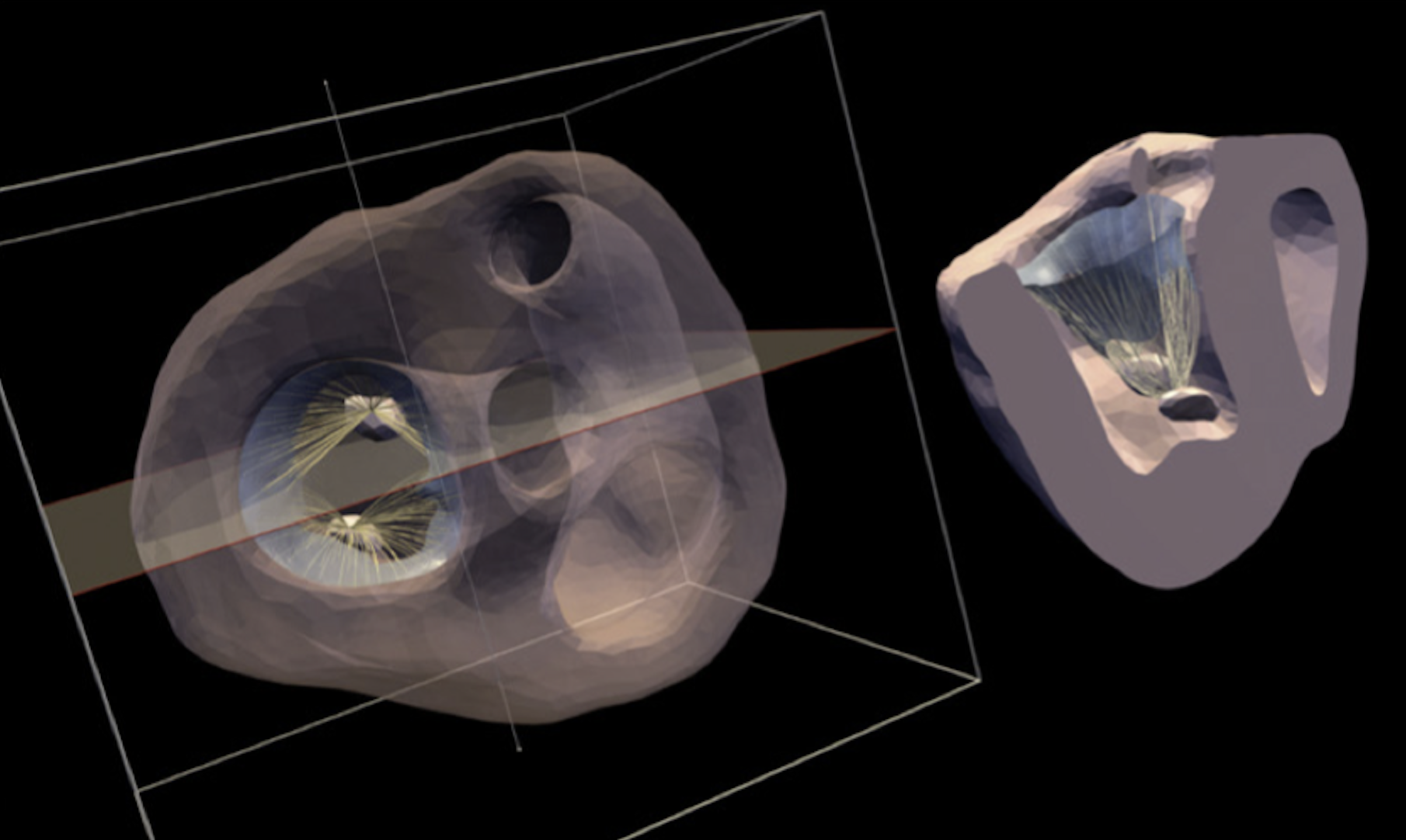
They started by using data from experiments involving sheep models of left heart failure to build this model, and then used the model to simulate what happens when someone has a heart attack, focusing on how the size and location of the heart infarcted (i.e. dead) tissue that results in mitral valve regurgitation.
Their simulations revealed how infarcted (and dead) heart tissue in different regions of the heart affect the mitral valve function. They found that particular locations of damage can change much worse regurgitation compared to other locations. Importantly, these findings were completely performed in-silico (that is, by a computer) and agreed extremely well with experimental findings. Understanding these finding can help doctors better diagnose and treat IMR, potentially improving outcomes for patients with this condition.
However, there is one major roadblock to getting these computational models in the hands of doctors…
In the wake of a heart attack, doctors need to make quick decisions and can’t always afford to wait hours or even days for a computer to analyze the situation.
“The fundamental problem with all these advanced simulations is that while they are great scientific tools, they are very slow,” explained Sacks. “We need new computational tools to make this work. Otherwise, it's great science, but it's never going to be used in the clinic."
Sacks and his team addressed this in their second paper by introducing a faster method for running simulations of the heart. Their innovation lies in rethinking how we solve the complex equations that describe heart function. Traditional models use the finite element method, which requires complex and time-consuming calculations for each scenario.
Instead, the team turned to machine learning. But rather than using the typical approach, which involves training an AI on massive datasets, they trained their AI on the physics of the heart—how factors like volume and pressure change as the heart beats.
By using these physics-based models, the researchers were able to maintain accuracy while drastically reducing computation time. They trained neural networks (machine learning models designed to mimic simple brain functions) to predict heart behavior based on fundamental physical principles used in the current heart models. This allowed the machine learning models to simulate an entire heartbeat in less than a second—something that would take hours with traditional methods.
The physics-based model is also highly adaptable. Traditional models need extensive recalibration for different scenarios, but the neural network-based method can quickly adjust to various heart conditions. For instance, it can simulate a complete heartbeat automatically, providing a detailed understanding of heart function specific to a given patient.
This new approach by Oden Institute researchers holds promise for heart attack patients suffering from IMR, with the potential to usher in a new era of cardiac care.